Table of Contents
Barrier membranes used in dentistry
Guided Tissue Regeneration (GTR) treatment is using cell-occlusive barrier membranes to exclude relatively rapid epithelial and fibroblastic downgrowth, while promoting repopulation of defect sites with slower migrating cells from the periodontal ligament, bone, and cementum.
Guided bone regeneration (GBR) is developing for the regeneration of osseous defects based on the principles as GTR, use of barrier membranes to exclude soft tissue ingrowth from the defect site to promote repopulation with osteogenic progenitor cells.
Resorbable membranes vs. Non-resorbable membranes
Both non-absorbable and bio-absorbable barrier membranes have been developed and modified in an attempt to be implanted over the tissue defect area to prohibit cell invasion from the gingival epithelium and connective tissue.
Non-absorbable membrane is commonly used for relatively large-scale tissue regeneration. Bio-absorbable membrane is exclusively polymer-based from both natural or synthetic material.
Bio-absorbable membranes have the advantages of few complications as well as secondary surgeries not being necessary, it is regarded a first-choice material in GTR or GBR treatment.
| Barrier membranes | 2nd surgery | Complications | Mechanic Strength | Biocompatibility | Wound Healing |
|---|---|---|---|---|---|
| Resorbable membranes | Not Necessary | Low Risk | Favorable | Good | Promote |
| Non-absorbable membranes | Need | High Risk | High | Good | No work |
Types of Bio-absorbable membranes
Bio-absorbable membrane can be divided into:
- natural
- synthetic material
Resorbable collagen membrane comprised of type I or type III collagens derived from bovine or porcine are the commonly used materials for natural polymer membranes.
Chitosan and alginate have also been introduced for use as barrier membranes in the study phase. Synthetic materials such as polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL) and their copolymers are also used as barrier membranes.
The Role of Resorbable Collagen Membranes in GTR and GBR Treatments
Collagen has excellent biocompatibility and capability on promoting wound healing, and it has been utilized in various medical applications such as wound dressing.
For both GTR and GBR treatments, resorbable collagen membranes have been shown to be comparable to non-absorbable membranes with regard to probing depth reduction, clinical attachment gain, and percent of bone fill. That is, resorbable collagen membranes is able to prevent epithelial downgrowth along the root surfaces during the early phase of wound healing, and to improve the bone generation of osseous defect by integrating with grafting material.
Revolutionizing Dental Regeneration: Maxigen Biotech Inc. (MBI)
Maxigen Biotech Inc. (MBI) has been dedicated to the development and application medical device of collagen and hyaluronic acid. MBI utilizes its core competence in biopolymers and has developed a broad range of biomedical applications in areas such as orthopedic, plastic surgery, wound management and dental regeneration. In the dental applications, the product line of MBI includes collagen based regenerative matrix such as GTR/GBR membrane and synthetic bone graft such as collagen/bio-ceramic composite.
Why resorbable collagen membrane is your best choice compared to other resorbable membranes
Several commercially available resorbable collagen membrane have been developed using type I collagen such as BioGide, BioMend Extend, FormaAid. Type I collagen is a pre-dominant component found in periodontal connective tissue, and the collagen component also possess additional advantages including hemostasis, chemotaxis for periodontal ligament (PDL) fibroblasts and gingival fibroblasts, and ability to augment tissue thickness. Hence, resorbable collagen membrane appears to be an ideal choice for an absorbable GTR or GBR barrier.
The resorption time for resorbable collagen membrane
The degradation rate of resorbable collagen membrane varies depending on the source and the implant site. Pitaru et al. reported a half-life of 21 to 28 days for non-crosslinked collagen when implanted intramuscularly or subcutaneously ref 1, however, there is no residual collagen material protruding from the gingival margin was noted at one week after Class II furcation defects treatment.
The ideal barrier membrane muse be able to exclude unwanted epithelial cells and maintain a space for repopulation of defect sites. Therefore, the barrier must maintain its structural integrity during early wound healing. For that purpose, various cross-linking techniques have been developed to prolong the absorption time of resorbable collagen membrane.
Excellent Resistance to Degradation
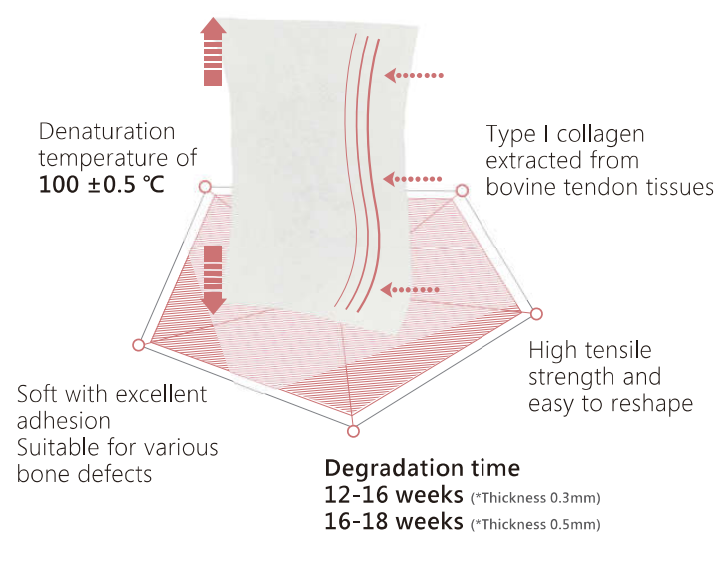
MBI FormaAid® features an excellent degradation time of approximately 12-18 weeks. Through a process of physical cross-linking, FormaAid® is designed to appropriately align with the rate of bone tissue regeneration. Furthermore, it exhibits similar anti-degradation properties when compared to other samples tested (bioxxx or BioXXX Extend).
Ref. 1: Pitaru S, Tal H, Soldinger M, Grosskopf A, Noff M. Partial regeneration of periodontal tissues using collagen barriers. Initial observations in the canine. J Periodontol 1988;59:380-386.
What types of dental procedures is resorbable collagen membrane used for?
Since the late 1980s, resorbable collagen membrane have been tested for their ability to promote regeneration in periodontal intrabony defects. Moreover, resorbable collagen membrane is utilized to promote regeneration in furcated molars for Class II furcation defects. Recently, resorbable collagen membrane have been used in root coverage procedures.
How is resorbable collagen membrane utilized for guided bone regeneration (GBR)?
Since resorbable collagen membrane can fulfill the requirements for an ideal GBR barrier including wound stabilization, space creation and maintenance, protection of the underlying blood clot, and the ability to exclude unwanted tissues or cells (i.e. connective tissue and epithelium), it has become one of the materials used in GBR procedures.
How is resorbable collagen membrane utilized for guided tissue regeneration (GTR)?
Collagen is an essential component of bone and connective tissue and supports these tissue structures. It is well known that various human cells can easily attach to the collagen membrane, and resorbable collagen membrane can promote the production of basic fibroblast growth factor (FGF)-2 and bone morphogenetic protein (BMP)-2 during cell proliferation and migration phase to improve the expected clinical outcome in the distinct application. Additionally, there are various methods to modify the such as micro-structure, porosity, hydrolysis or enzyme resistance, suturing capability of resorbable collagen membrane to achieve the clinical requirements in GTR procedure.
MBI FormaAid®Collagen Membrance GTR Membrance With Superb Stretch& Tear-resistance
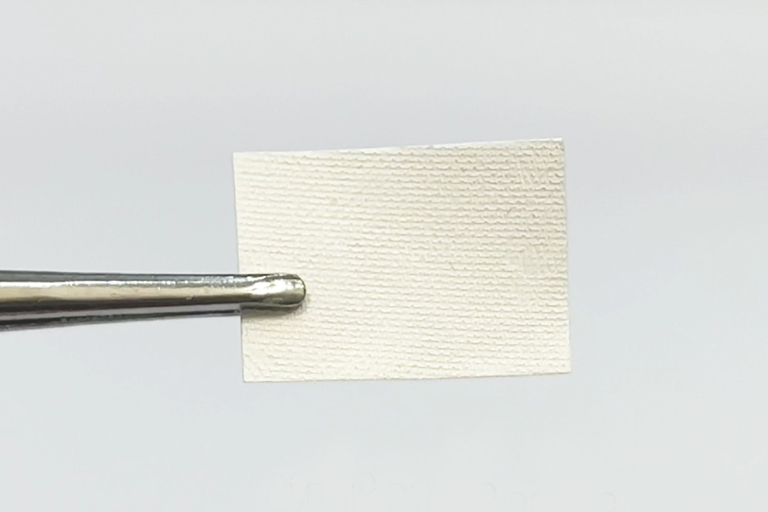
MBI FormaAid Collagen Membrane is a resorbable collagen membrane fabricated by highly purified fibrillar collagen. FormaAid Collagen Membrane has high stretchable characteristic without chemical crosslinking.
Therefore, FormaAid® Collagen Membrane exhibits ideal properties extremely suitable for handling, suturing and barrier functionality during GTR or GBR procedure. FormaAid® Collagen Membrane is indicated for use in the surgical treatment of periodontal defects to aid in the regeneration and integration of tissue components in GTR or GBR procedures to enhance wound healing.
Advantages of MBI FormaAid®
- High-strength support! Preserving collagen’s triple-helix structure with physical cross-linking technology.
- Natural collagen extracted from non-epidemic zone Australian cattle tendon tissues.
- High-purity Type 1 Collagen crafted with US-patented purification techniques.
- Porosity and fibrous structure is ideal for rapid tissue regeneration.
- High tensile strength and excellent flexibility for diverse bone defects in clinical settings.
- Diverse specifications for various oral wound sizes.
How long does it take for resorbable collagen membrane to dissolve?
Factors which can lead to collagen degradation include ageing, disease or exposure to UV radiation. However, the main cause to degrade collagen in vivo is enzyme reaction. The major enzymes for collagen degradation are collagenases, which belong to a group of enzymes called matrix metalloproteinases (MMPs). Collagenases are released dominantly by inflammatory cells including macrophages, neutrophils and tumor cells.
On the other hand, the degradation behavior of resorbable collagen membrane will also depend on its physical and chemical properties caused by such as collagen purification process, porosity of the matrix, chemical crosslinking or not, etc. In general, the degradation time of resorbable collagen membrane may be varied from weeks to months.
Possible side effects associated with using resorbable collagen membrane:
Currently, all available resorbable collagen membrane are comprised of animal source collagen from such as bovine or porcine. Patients with a history of allergy to any animal source collagen products may cause hypersensitivity. Signs and symptoms of local allergic reaction are pain (at the wound site) and localized redness that may extend beyond the perimeters of the wound site. Besides above hypersensitivity reactions, the potential side effect such as irritation or edema may be caused by residual chemical crosslinker in the resorbable collagen membrane with chemical crosslinking.
MBI Collagen Membrane (FormaAid® ) features with physical cross-linking, offering excellent clinical maneuverability and conformability. It provides ample biological barrier time to guide soft tissue growth, thereby maintaining the alveolar bone space to facilitate bone tissue regeneration. This approach mitigates the risk associated with residual chemical cross-linking agents, ensuring a higher level of safety and improved biocompatibility.
How to minimize possible side effects caused by using resorbable collagen membrane?
Resorbable collagen membrane must not be used by patients with a history of allergy to any animal source products. From clinical using aspect, it is not recommended to use resorbable collagen membrane in the infected area of a wound site. From manufacturing aspect, resorbable collagen membrane with chemical crosslinking will raise higher risk in inflammatory reaction followed by difficult wound management or faster resorption.
MBI Pioneering Approach to Collagen and Hyaluronic Acid-Based Devices
Maxigen Biotech Inc., as a specialist in the medical device industry, offers innovative products from five disciplines and is certified by CE, US FDA, NMPA, KFDA product certifications of major countries in worldwide.
MBI are committed to using science to join and delight human life. MBI has been dedicated to the development and application medical device of collagen and hyaluronic acid since 1998. MBI utilizes its core competence in biopolymers and has developed a broad range of biomedical applications in areas such as orthopedic, plastic surgery, wound management and dental regeneration.
Every year, there are more than 3 million units medical device manufactured by MBI are using in worldwide. Join MBI to delight human life.
MBI commitment to advancing healthcare is truly commendable. With a strong foundation in the dental medical device industry and a passion for improving human life, MBI stands at the forefront of innovation. In addition to resorbable collagen membranes, FormaAid®Collagen Membrane, MBI also offers resorbable collagen plugs, HealiAid® Collagen Wound Dressing, which is the best choice for oral soft tissue repair. Further reading: Healing Power of Collagen Wound Dressing: A Comprehensive Review
What are the key advantages utilizing a resorbable collagen plug?
Resorbable collagen plug is a cylindrical-shaped collagen matrix for fitting in the extraction socket, it can provide a scaffold for periosteal cell growth which is suitable for applications in bone tissue engineering. That is, the resorbable collagen plug can keep excellent healing quality of tooth extraction socket to prevent resorption of the alveolar bone which is eventually causing clinical difficulties for future implant-supported procedures. Therefore, the socket preservation using resorbable collagen plug is an effective strategy to maintain bone volume after tooth extraction.
What are the limitations of utilizing a resorbable collagen plug?
Resorbable collagen plug wound dressing is designed as a cylindrical-shaped collagen matrix for fitting in the extraction socket, and it can absorb many times its own weight in exudate. Therefore, it is not recommended to compress the matrix for fitting other types of wound before using. Additionally, patients with a history of allergy to any animal source collagen products is not recommended to use any collagen product comprised of animal source collagen from such as bovine or porcine.